 |
|||
|
Page Title:
Barometric pressure temperature correction (variable C) |
|
||
| ||||||||||
|
|  MIL-C-48868E (AR)
Tap the check valve to help it open. Allow the gas to
equilibrate thermally with the water in the jacket around the
eudiometer tube. Wait at least 20 minutes. Measure the volume
of gas collected in the audiometer tube by uncorking the
leveling bulb and adjusting it so that the top of the fluid in
the bulb is at exactly the same height as the top of the fluid
in the audiometer tube. Read the volume to the nearest
0.1 ml. Use the following equation to calculate the percent NC
in the sample:
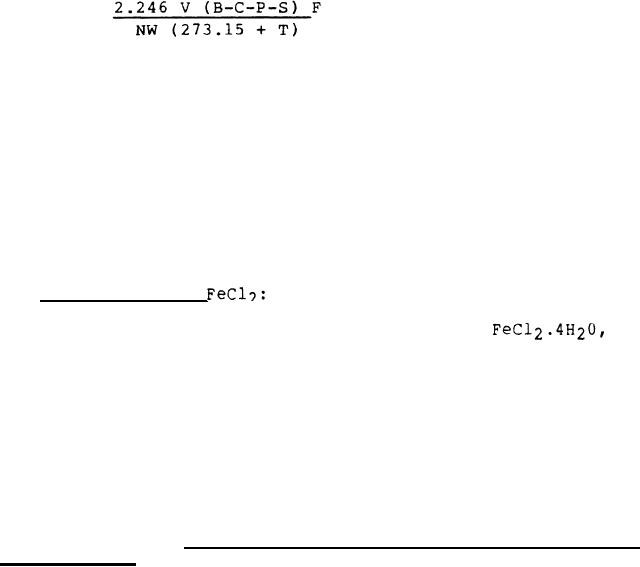
% NC =
V = Volume of gas in audiometer tube (ml)
B = Barometric pressure (mm Hg)
C = Temperature correction (mm Hg), see paragraph
4.5.1.3.3.2
P = Vapor pressure of water over 33% (w/w) NaOH at T
(mm Hg), see paragraph 4.5.1.3.3.2
F = Audiometer factor
N = Percent nitrogen in NC (%)
W = Mass of sample (g)
T = Temperature of condenser water (C)
S = Local gravity & sea level values corrected to standard
values
PREPARATION OF
Prepare the solution by adding 1000 g of
1200 ml deionized water and 70 ml of concentrated HCl to a 5
liter round bottom flask. Attach the reflux condenser and
reflux for 20 minutes. Cool to room temperature. Slowly add 20
grams of iron fillings or powder to the cool solution. When the
gas evolution has stopped, filter quickly without aerating the
solution to remove the fines. Use A.C.S. grade chemicals
throughout the synthesis.
Prepare the bath fluid by dissolving reagent grade NaOH in
deionized water until the concentration is 33% weight to weight.
4.5.1.3.3.2 Barometric pressure temperature correction
(variable C). The value for variable C is obtained by using t h e
barometric pressure (mm Hg) and the barometric thermometer
temperature (C) with the following:
24
|
|
Privacy Statement - Press Release - Copyright Information. - Contact Us |