 |
|||
|
Page Title:
Hydroxylamine hydrochloride solution |
|
||
| ||||||||||
|
|  MIL-P-670A
(c) Procedure.
Weigh a 1 gram sample into a 400 ml. beaker,and
add 10 ml. of water and 35 ml. of nitric acid.
Carry along a reagent blank.
Boil down to a volume of about 20 ml. and add 2 ml. of perchloric acid.
Evaporate to fumes of perchloric acid, and fume for 1 to 2 minutes. cool
to room temperature.
Add 25 ml. of water, 5 ml. of ammonium citrate
solution (40 percent) and 1 ml, of EDTA solution (5 percent), and proceed
as described in paragraph 4.5.3 (b).
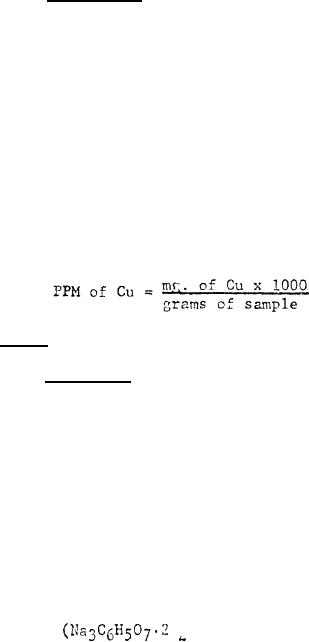
Convert the reading to mg. of
copper by consulting the calibration curve,
Calculate as follows:
4.5.4 Iron.
(a) Reagents.
Hydroxylamine hydrochloride solution (5 percent) . Dissolve
25 grams of hydroxylamine hydrochloride in water and dilute to 500 ml.
Ortho-phenanthroline solution (0.2 percent). Dissolve 1
gram of ortho-phenanthroline in water and dilute to 500 ml .
Store in a
dark bottle.
Sodium citrate solution (20 percent).
Dissolve 100 grams of
in water and dilute to 500 ml.
sodium citrate
Standard iron solution (1 ml. = 0.1 mg. of Fe).
Dissolve
0.1000 gram of pure iron (National Bureau of Standards Sample 55c) in 50 ml.
of hydrochloric acid by heating on the hot plate in an Erlenmeyer flask.
Add 3 ml. of hydrogen peroxide (30 percent), boil 10 minutes, cool, and
dilute to 1 liter in a volumetric flask,
12
|
|
Privacy Statement - Press Release - Copyright Information. - Contact Us |