 |
|||
|
|
|||
| ||||||||||
|
|  MIL-C-48868E (AR)
Distillation.
Before turning on the distillation unit, make sure that the
tap water is on. The NaOH reservoir and
reservoir should
be checked and filled with a solution of 102 g/l sodium
thiosulfate in 10N NaOH (1:5). Depress on/off switch so that
the green light comes on. Check to see if proper water
circulation occurs. (Large rubber return line should have
water flowing through it.) NOTE: If flow rate through this
tube is too high, the unit will not operate, it will blow a
fuse. Check the operation manual, page 13, instructions 5.1 to
insure proper flow rate. Preheat the Buchii unit with about
for five minutes. Wet the distil-
100 mls of distilled
lation system connector with water and place the reaction flask
in the unit. Place receiving flask, with 25 mls boric acid
(40 g/l) under collection tube, so that the tube is below the
liquid level and close the shield. Depress the distillation
botton and let unit distill until 100 mls of distillate is
collected.
Remove collection flask from tube. Let the
collection tube drip the remainder of distillate into the boric
acid solution. Remove reaction flask from the stand. Pour
contents into the nearest acid drain.
Analysis.
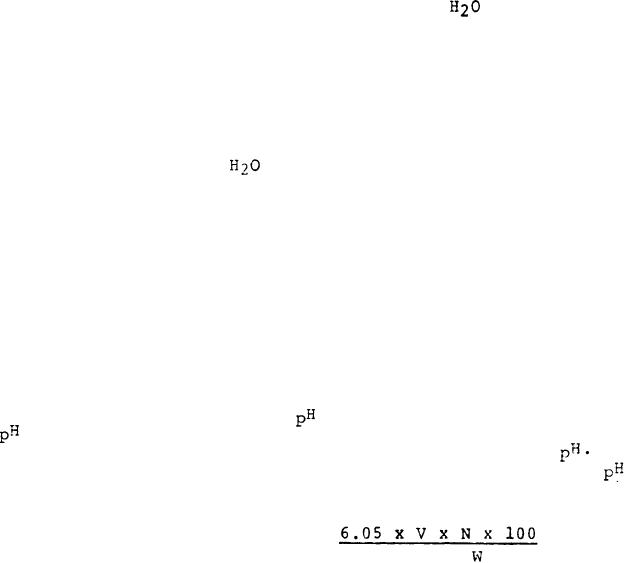
Drain the 10 ml buret twice with standardized 0.1N HCl
solution. Standardize the
meter and electrode using
4 and H 7 buffer solutions. Titrate the ammonia-boric
acid complex with the standard 0.1N HCl to a 4.65
At
least two blanks (complete digestion, distillation and
measurement) are to be performed every week. Calculate the
percent of acrylic fibers in the specimen as follows:
Percent acrylic fibers =
Where,
V = Volume of standard HCl for sample, ml
N = Normality of standard HCl
W = Weight in grams, of original sample
31
|
|
Privacy Statement - Press Release - Copyright Information. - Contact Us |